45 olestra warning
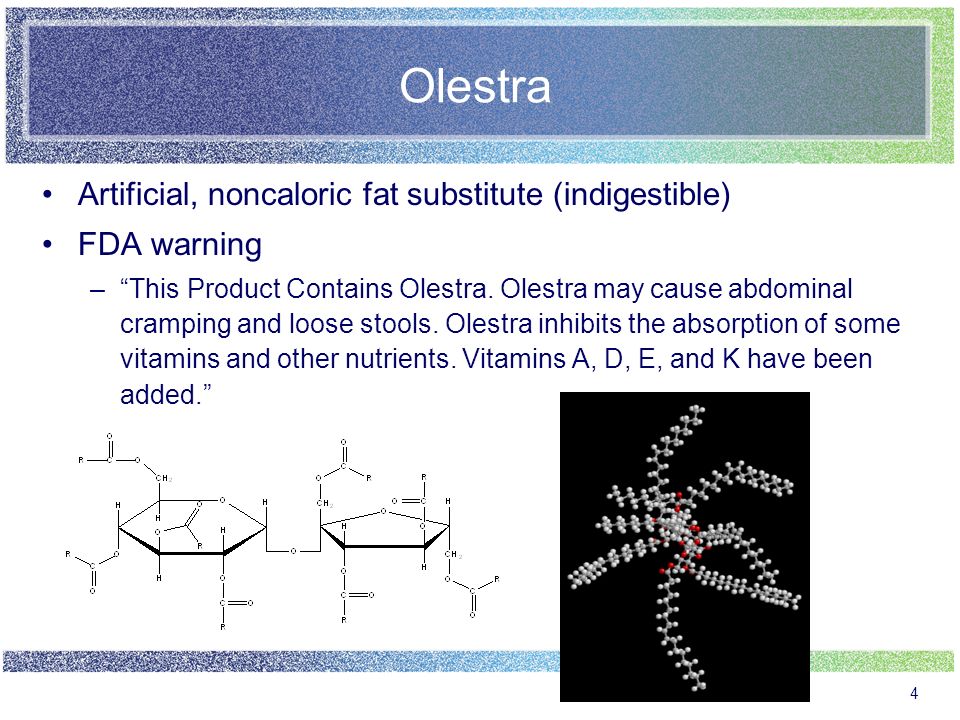
The Potato Chip That Destroyed The Bowels Of America Sep 25, 2020 · Olestra had actually been invented back in the 1960s, by some well-meaning Procter & Gamble researchers trying to find a way to quickly deliver fat to underweight babies. They screwed this up so badly that they actually invented a new kind of fat that the human body was completely incapable of absorbing. Lay's WOW chips - Wikipedia Although initially popular, charting sales of $400,000,000 in their first year, they subsequently dropped to $200,000,000 by 2000, as Olestra caused "abdominal cramping, diarrhea, fecal incontinence ["anal leakage"], and other gastrointestinal symptoms" in some customers, warnings were required to be included on the packaging, with the WOW bag bearing a warning that read, "This Product Contains Olestra. Olestra may cause abdominal cramping and loose stools.
Olestra Side Effects - Nutrineat Gastrointestinal Problems: Over consumption of products that contain this addictive can lead to many gastrointestinal issues, like abdominal cramps, diarrhea, and loose stools (this condition is also known as anal leakage). It may also lead to fatigue and nausea, followed by vomiting.

Olestra warning
melmagazine.com › en-us › story‘Wow! These Chips Just Made Me Crap My Pants’: The Olestra Story To get olestra approval from the FDA, PG took a page from the tobacco industry's lobbying playbook, per a Harvard University review. Lawmakers from both sides of the aisle who had received money from PG's Political Action Committee wrote a joint letter in 1995 to the Secretary of Health & Human Services, arguing that olestra was safe and should be approved by the FDA. Olestra - Cnn April 10 -- Health Briefs: Olestra makers ask FDA to tone down warning labels; July 2 -- Health Briefs: Fat substitute may be bad for health August 25 -- New fake fat: tastes good, less filling New olestra complaints bring total close to 20,000 - more than all ... WASHINGTON— The Center for Science in the Public Interest (CSPI) today forwarded to the Food and Drug Administration (FDA) more than 200 new complaints of adverse reactions from consumers who had eaten snack foods containing the indigestible fat substitute olestra. With close to 20,000 reports forwarded to the agency from both CSPI and olestra developer Procter & Gamble, the FDA has logged more complaints about olestra than it has about all other food additives in history combined.
Olestra warning. Olestra warning!! - YouTube ignorejust uploaded for a chem. project.. › world-view › list-foods-made-olestra-5adWhat Is a List of Foods Made With Olestra? - Reference.com Mar 27, 2020 · Foods made with olestra include all of Lay’s light potato chip products and Pringles’ fat-free potato chips. Olestra, also called by its brand name Olean, was chosen as an ingredient in these snacks because it’s a fat substitute that adds no fat, calories or cholesterol to food products. There are two popular brands using olestra in their potato chips: Lay’s and Pringles. content.time.com › time › specialsOlestra - The 50 Worst Inventions - TIME May 27, 2010 · In January 1996, the FDA approved olestra as a food additive. Cut out the unhealthy cooking oil. Shred the package of shortening. Bury the stick of butter. Frito-Lay was among the first companies... Olestra: The Embarrassing Story Behind the Once-Famous Fat … Olestra: The Embarrassing Story Behind the Once-Famous Fat Substitute. In the mid-1990s, fat was not the food hero as it is seen nowadays. Foods with a high fat content were shunned from the kitchen, and from our diets too. But as people are, we still love to snack on tasty treats, so it’s no surprise that food manufacturers came out with the new wonder ingredient – Olestra!
FDA removes Olestra warning - NBC News The Food and Drug Administration lifted the warning Friday, concluding that if the zero-calorie fat substitute has any stomach-troubling effect, it's mild and rare. The FDA approved olestra's... The Potato Chip That Destroyed The Bowels Of America However, they insisted on one condition -- the new products all had to have a prominent label warning that Olestra could cause abdominal cramping, fecal incontinence and loose stools. And stools are like timberwolves: certainly an important part of the ecosystem, but you really don't want a loose one appearing when you put out a bowl of chips at a cocktail party. Those gut-wrenching Olestra chips from the '90s might have ... - Quartz Olestra, which was marketed under the brand name Olean, was a dieter's dream when it was marketed in the 1990s, during the low-fat craze. It was also a massive pain—in the gastrointestinal area,... What Were They Thinking? The Chips That Sent Us Running To The Loo At first glance, olestra seemed like a dieter's dream. While it provided the satisfaction of tasting just like fat, its molecules were too large to be digested by the body, passing directly...
Product Fail. WOW! CHIPS, FRITO-LAY (1998) - Medium This line of chips was made with Olestra, an artificial fat that was supposed to pass harmlessly through your digestive tract. Gastrointestinal side effects of an unmentionable variety ensued ... Olestra Warning No Longer Required\ the Fda Says the Fat Substitute ... The Food and Drug Administration lifted the warning Friday, concluding that if the zero-calorie fat substitute has any stomach-troubling effect, it's mild and rare.The FDA approved olestra's sale ... › article › 619261Olestra Fat-Free Snack Controversy of the 1990s | Mental Floss The agency also mandated a package warning about abdominal cramping and loose stools, an observed side effect of olestra consumption. Procter & Gamble made a minor stir about the label—after... What Is a List of Foods Made With Olestra? - Reference.com Mar 27, 2020 · Foods made with olestra include all of Lay’s light potato chip products and Pringles’ fat-free potato chips. Olestra, also called by its brand name Olean, was chosen as an ingredient in these snacks because it’s a fat substitute that adds no fat, calories or cholesterol to food products. There are two popular brands using olestra in …
› food-recipes › newsOlestra Eaters, Beware - WebMD Feb 14, 2000 · Olestra was approved in January 1996 by the FDA for use in place of fats and oils in prepackaged savory snacks. Olestra aims to reduce a food's fat and calories while maintaining its texture....
10 Foods Americans Eat That Are Banned Around the World (Slideshow) Olestra is a zero-calorie fat substitute created to make healthier snacks such as fat-free potato chips. But olestra has been shown to cause side effects in the form of gastrointestinal problems, as well as weight gain — instead of weight loss — on lab rats. The U.K. and Canada are two places that have banned this fat substitute from their food markets.
Olestra - Wikipedia Olestra is a fat substitute that adds no calories to products. It has been used in the preparation of otherwise high-fat foods thereby lowering or eliminating their fat content. The Food and Drug Administration originally approved olestra for use in the US as a replacement for fats and oils in prepackaged ready-to-eat snacks in 1996, concluding that such use "meets the safety standard for food additives, reasonable certainty of no harm".: 46399 In the late 2000s, olestra lost its …
en.m.wikipedia.org › wiki › LayLay's WOW chips - Wikipedia Although initially popular, charting sales of $400,000,000 in their first year, they subsequently dropped to $200,000,000 by 2000, as Olestra caused "abdominal cramping, diarrhea, fecal incontinence ["anal leakage"], and other gastrointestinal symptoms" in some customers, warnings were required to be included on the packaging, with the WOW bag bearing a warning that read, "This Product Contains Olestra. Olestra may cause abdominal cramping and loose stools.
‘Wow! These Chips Just Made Me Crap My Pants’: The Olestra Story Feb 8, 2022 · Olestra, also known by its brand name Olean, had become shorthand for a nation that had lost its way. In 1997, Procter & Gamble was about to launch its new fat-free snack foods via Nabisco and Frito-Lay. To see if Americans would munch on the chips fried in calorie-free olestra, they made Indianapolis their open-air testing ground.
Olestra - The 50 Worst Inventions - TIME May 27, 2010 · In January 1996, the FDA approved olestra as a food additive. Cut out the unhealthy cooking oil. Shred the package of shortening. Bury the stick of butter. Frito-Lay was among the first companies...
How does olestra work? | HowStuffWorks Dec 4, 2000 · Olestra is a fat substitute. It is found in a number of snack foods, from potato chips to frozen desserts. In these products, you'll find it in the ingredient list under its brand name, Olean. Chemists create olestra by combining two naturally occurring substances, sucrose and vegetable oil, to form a molecule that is not found anywhere in nature.
recipes.howstuffworks.com › question526How does olestra work? | HowStuffWorks Dec 4, 2000 · Olestra is a fat substitute. It is found in a number of snack foods, from potato chips to frozen desserts. In these products, you'll find it in the ingredient list under its brand name, Olean. Chemists create olestra by combining two naturally occurring substances, sucrose and vegetable oil, to form a molecule that is not found anywhere in nature. Yet the resulting synthetic molecule tastes just like real fats do!
PDF Re: The Urgent Need for Warning Label on Synthetically Dyed Foods to ... In regards to olestra, the FDA's principal concern in requiring a warning label was not related to the severity of the symptoms caused by its ingestion. In fact, the FDA repeatedly stated that it found "no safety concerns with respect to the effect of olestra on the gastro-intestinal (GI) tract." 9. FDA stated:
Olestra: The Embarrassing Story Behind the Once-Famous Fat ... - Cookist Olestra was discovered (almost by accident) by Procter and Gamble researchers. While exploring different fats that are tolerable for infants, they discovered that fatty acids attached to a certain sugar alcohol (sorbitol), makes the molecule so big it cannot be absorbed through the intestines. This makes it indigestible.
Olestra Eaters, Beware - WebMD Feb 14, 2000 · Olestra was approved in January 1996 by the FDA for use in place of fats and oils in prepackaged savory snacks. Olestra aims to reduce a food's fat and calories while maintaining its texture....
Those gut-wrenching Olestra chips from the ’90s might have Apr 9, 2014 · Olestra, which was marketed under the brand name Olean, was a dieter’s dream when it was marketed in the 1990s, during the low-fat craze. It was also a massive pain—in the gastrointestinal area,...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Olestra, as identified in this section, may be safely used in accordance with the following conditions: (a) Olestra is a mixture of octa-, hepta-, and hexa-esters of sucrose with fatty acids derived from edible fats and oils or fatty acid sources that are generally recognized as safe or approved for use as food ingredients.
Olestra - Wikipedia Olestra is a fat substitute that adds no calories to products. It has been used in the preparation of otherwise high-fat foods thereby lowering or eliminating their fat content. The Food and Drug Administration originally approved olestra for use in the US as a replacement for fats and oils in prepackaged ready-to-eat snacks in 1996, concluding that such use "meets the safety standard for food additives, reasonable certainty of no harm".: 46399 In the late 2000s, olestra lost its popularity due
Olestra To Lose Gastrointestinal Warning | American Council on Science ... Olestra To Lose Gastrointestinal Warning. By ACSH Staff — August 5, 2003. Products containing Olestra, the zero-calorie fat substitute, will no longer bear a label informing consumers of purported unpleasant gastrointestinal (GI) side effects. The Food and Drug Administration (FDA), after reviewing a six-week study that involved 3000 people, ruled that Olestra "caused only mild, infrequent GI effects," according to an FDA press release.
New olestra complaints bring total close to 20,000 - more than all ... WASHINGTON— The Center for Science in the Public Interest (CSPI) today forwarded to the Food and Drug Administration (FDA) more than 200 new complaints of adverse reactions from consumers who had eaten snack foods containing the indigestible fat substitute olestra. With close to 20,000 reports forwarded to the agency from both CSPI and olestra developer Procter & Gamble, the FDA has logged more complaints about olestra than it has about all other food additives in history combined.
Olestra - Cnn April 10 -- Health Briefs: Olestra makers ask FDA to tone down warning labels; July 2 -- Health Briefs: Fat substitute may be bad for health August 25 -- New fake fat: tastes good, less filling
melmagazine.com › en-us › story‘Wow! These Chips Just Made Me Crap My Pants’: The Olestra Story To get olestra approval from the FDA, PG took a page from the tobacco industry's lobbying playbook, per a Harvard University review. Lawmakers from both sides of the aisle who had received money from PG's Political Action Committee wrote a joint letter in 1995 to the Secretary of Health & Human Services, arguing that olestra was safe and should be approved by the FDA.





































Post a Comment for "45 olestra warning"